Details of the Drug
General Information of Drug (ID: DM8B9YT)
| Drug Name |
Ro-31-4724
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Ro-31-4724; CHEMBL92608; [[1-[N-HYDROXY-ACETAMIDYL]-3-METHYL-BUTYL]-CARBONYL-LEUCINYL]-ALANINE ETHYL ESTER; RO4; 112105-54-1; Ro 314724; ro 31-4724; AC1NUPD6; ZINC3801503; ro-314724; BDBM50146631; DB08482; RO314724; (R)-N-(N-(2-(2-(Hydroxyamino)-2-oxoethyl)-4-methyl-1-oxopentyl)-L-leucyl)-L-alanine ethyl ester; ethyl N-{(2R)-2-[2-(hydroxyamino)-2-oxoethyl]-4-methylpentanoyl}-L-leucyl-L-alaninate; ethyl (2S)-2-[[(2S)-2-[[(2R)-2-[2-(hydroxyamino)-2-oxoethyl]-4-methylpentanoyl]amino]-4-methylpentanoyl]amino]propanoate
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
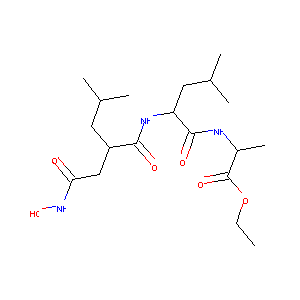 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 401.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 13 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
